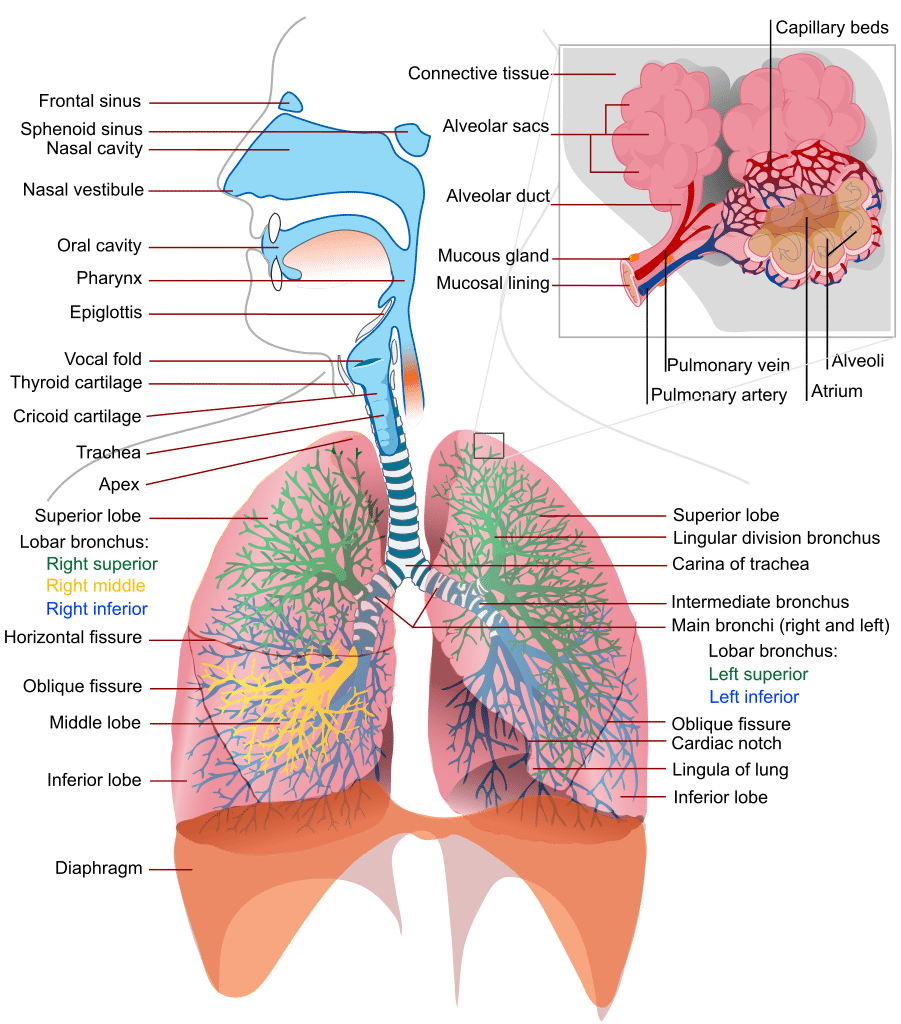
One of the myths that the fitness industry refuses to kill off is that breathing occurs primarily to provide oxygen to the body. OK, yes, we all know it’s true that breathing provides oxygen to the body. But that’s not the the primary function per se, it’s not the whole story and it’s not even the interesting part. Then we get into the outright falsehoods. For example, the idea that breath rate increases as the demand for oxygen increases is completely false. It seems like common sense, but it’s still false. The fact is that our bodies don’t consider oxygen at all when it comes to breathing. That probably sounds crazy but it’s true. To understand why, we need to understand exactly how breathing actually works.
First we must understand the dual role that the respiratory system plays. The purpose of the respiratory system is the exchange of two gasses: oxygen and carbon dioxide. Most people understand the oxygen piece very well, so let’s move into something less known and kill the myth of oxygen deficiency. The only time there is an actual oxygen deficiency at the muscle cell is when the muscle is swollen from active contraction greater than 15% of 1-repetition maximum (1RM) or injury to the point where the inflammatory response occludes the capillaries. In such instances there is a temporary period of hypoxia. That is normal, not a big deal and doesn’t constitute a true oxygen debt. True hypoxia at the cellular level is something you will most definitely experience in certain situations such as being newly arrived at high altitude. Please put that myth away so that you can understand the true power of the respiratory system.
The more important and profound function of the respiratory system is the regulation of blood acidity or “pH” via the expulsion of carbon dioxide. The reason this function is more important is because the very act of breathing itself and at what rate and depth, is determined by carbon dioxide and blood acidity, not oxygen demand!
Many have heard of pH but few know what it actually is. “pH” is an abbreviation that stands for “partial pressure of hydrogen” and it’s not a gas, but a measurement of acidity or alkalinity, based on the hydrogen (H+) ions present. The pH of a solution is equal to the negative log of the hydrogen ion concentration in that solution: pH = – log [H+]. The normal blood pH depends on which side of the capillary beds we’re discussing. The pH range for arterial blood is 7.35-7.45. The pH range for venous blood is 7.32-7.42. The body attempts to keep arterial blood just above 7.4 (more alkaline) and venous blood just under 7.4 (more acidic).
When you exercise, carbon dioxide (CO2) leaves the muscle cell and dumps into the veins via the capillaries. This increases the acidity of the venous blood and the pH level drops. When this more acidic blood hits chemoreceptors that line the major blood vessels, it signals the brain and the signal is returned to increase breath rate and depth to reduce the acid level of the blood quickly. At the same time, the heart rate is increased for the same reason: get that acidic blood to the lungs so the CO2 can get scrubbed out! Fortunately, the lungs are amazing CO2 scrubbers and they have the ability to scrub as much and more CO2 than we can produce.
But how exactly does oxygen enter the blood and CO2 leave it? We all understand that we inhale air into our lungs and that there are capillary beds around the grape bunch looking alveoli. But what is the process that actually makes these gasses enter or depart the blood itself? The answer is “diffusion” and it requires a difference in the “partial pressures” of a gas. Partial pressure is a fancy term for: the amount of a gas by weight in solution. For example, the amount of oxygen in the air at sea level is 21%. But we measure the partial pressure of oxygen at sea level and express it as 160 millimeters of mercury (mmHg). That is because oxygen has weight and we are able to measure it and assign a number based on the amount of mercury it displaces. This pressure steps down with each step it takes. For example, in the alveoli the pO2 is 100mmHg. In arterial blood it’s 80-100mmHg. Having used a bunch of oxygen in the cells, venous blood weighs in with a mere 40-50mmHg and by the time it hits the lungs again it’s a mere 20-40mmHg.
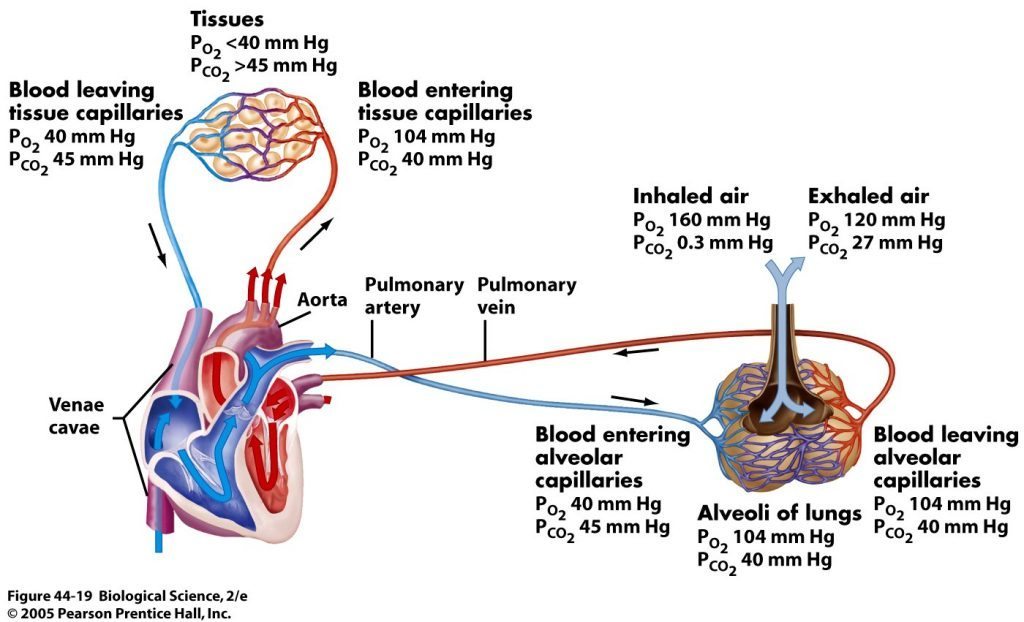
This sets us up perfectly to understand the process of gas diffusion. Diffusion is the spontaneous movement of gases, without the use of any energy or effort by the body, between the alveoli and the blood in the capillaries of the lungs. The rule with diffusion is that molecules move from areas of higher concentration to areas of lower concentration in an effort to balance concentrations or meet a saturation requirement. When that venous blood containing only 20-40mmHg hits the alveolar capillaries in the lungs, there is 100mmHg of oxygen on the other side of a 1 micron thick semi-permeable membrane. The oxygen rushes across the membrane and attaches to the hemoglobin on red bloods cells. The blood then departs the lungs at approximately 100mmHg. The opposite happens for CO2. The venous blood is packed with twice as much CO2 as the air in the lungs. Thus, CO2 rushes out of the blood and into the lungs. We can speed up this process with hard exhalations which reduce the concentration of CO2 in the alveoli even further and speeds the rate at which CO2 leaves the blood via diffusion.
That brings us to a fun point. If I told you to take five breaths as deep and as fast as you could, well, I’ve never found anyone who would do it. Why not? Because everyone knows you get dizzy and might pass out. But why? Is it due to a lack of, or too much oxygen? Nope! It’s because to do this rids the body of too much CO2 and the blood turns too alkaline. This is a very bad thing and when the body senses this change; the brain pulls the plug on itself. For one, it stops breathing because that halts the expulsion of CO2 by building up the amount of CO2 in the lungs and the blood. Often this is associated with a loss of consciousness or close to it. A real life example is a person who is hyperventilating. The cure is to have them breathe in and out of a paper bag. Why? So they will breath in the CO2 they are exhaling. This doesn’t necessarily calm them down, it just prevents them from passing out. Of course, if they pass out then they are no longer under the psychological spell and they will quickly return to normal.
This example illustrates why rate and depth of breathing is important. Hyperventilation is excessive ventilation. It is characterized as the excessive reduction in CO2 via excessive rate and depth (volume of air exchanged per breath cycle) of breathing. However, one must understand that the real story here is the volume of air exchanged in the breath cycle. Normal breathing moves 5-8 liters of air in and out of the lungs every minute. It doesn’t matter if that is done with a few deep breaths or many rapid shallow breaths. The body will still absorb and expel the oxygen and CO2 required to maintain proper pH levels. However, if a rapid rate changes from shallow to deep, the amount of air exchanged can go as high as 100L per minute. If the body is not dumping CO2 into the blood stream fast enough to justify this rate AND depth, hyperventilation is occurring.
On the flip side, hypoventilation (inadequate ventilation) is what drives sudden shifts in respiratory rate and depth. We have all heard the term “catch your breath”. This refers to the state where your breathing has not yet adequately restored your venous pH levels. A simple example is a short sprint. You take off but you don’t feel any need to breathe more. Then suddenly you gasp and you’re out of breath with a racing heart! Why? Because you executed the movement, then the blood was contaminated, then it had to move to the heart and lungs and only then was the presence of excess acid detected. The breathing mechanism is delayed 2-4 seconds from the reality of present blood pH levels and when it hits you it can hit hard!
Another example of hypoventilation can be seen when you perform a difficult physical task like running 3 kilometers as fast as you can. At the end of this event it takes you many minutes to “catch your breath” and for your heart rate to return to normal. The reason is two-fold. You were exercising at a rate that dumped excessive amounts of CO2 into your blood, and at a rate faster than you could breathe it away. Thus, when the event is over, you still have to scrub that CO2 out. Thus, your heart rate and breath rate (and depth) remain high until the pH level returns to normal.
Incidentally, there is a larger learning point I simply cannot resist here. The more “fit” you are for the task, the faster you will recover from such an event. If we use the 3km run for fastest time, no matter how fit you are, you will have excess CO2 that you cannot off-gas during the run. However, the amount you have depends on your fitness level. If you are properly trained, your muscles and physiology are dramatically more efficient. Yes, in a max effort there will be excess CO2, but only a fraction of an unfit person. You will also be able to repeat the performance whereas an unfit person is done for the day, perhaps the week. You can see this in advanced runners who return to normal within 1-3 minutes vs. those who still look like a truck hit them 10 minutes later.
Now for some homework! In the comments below, share a breathing experience you’ve had in exercise, a fight or combat that relates to the material above.